|
D-Transposition of the Great Arteries
This is the second most common congenital heart defect found in early infancy with a relative prevalence rate of approximately 0.24 per 1,000 live births (Keane, Chapter 37), accounting for 5% of cases of congenital heart diseases.
Anatomical description
D-TGA exists when the aorta arises from the morphologic right ventricle and is found anterior and rightward of the pulmonary artery. The pulmonary artery arises from the morphologic left ventricle and is found leftward and posterior with respect to the aorta. VSDs are commonly associated with D-transposition of the great arteries and present in approximately 35% of cases at birth (Keane).
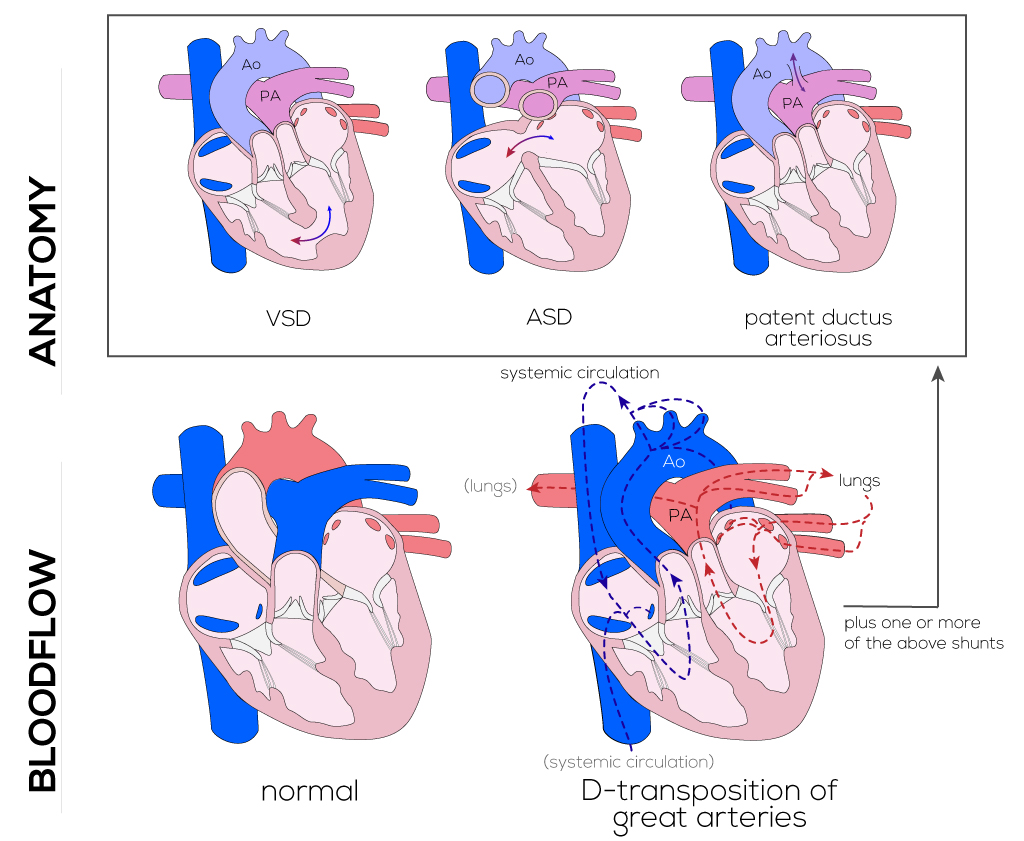
(Click picture to show/hide bloodflows)
Pathophysiology
Transposition of the great arteries results in two parallel circulations in which systemic venous (deoxygenated) blood passes through the right heart to the aorta and back to the systemic circulation. Highly oxygenated blood passes from the lungs to the pulmonary veins to the left ventricle, pulmonary artery, and back to the lungs. Since survival requires mixing of these parallel circulations, a communication exists in the form of an ASD or persistent ductus arteriosus. In either case, the amount of blood flowing into and out of the pulmonary circulation must be equal or greater than that in the systemic circulation.
Clinical manifestations of D-transposition of the great arteries depend, in part, on whether the ventricular septum is intact. Infants born with an intact ventricular septum may show cyanosis within hours of birth, while those with a VSD may show little or no cyanosis at all. Tachypnea is generally present and varies in timing of onset and severity, depending on the degree of pulmonary resistance or stenosis. Additional complications in these individuals commonly include respiratory distress and congestive heart failure, which may also develop within days of birth.
Therapy
Management of the transposition patient includes medication and/or surgical intervention. Prostaglandin E is given to maintain a persistent ductus arteriosus and allow left-to-right shunting when a VSD is not present. If the patent foramen ovale is restrictive, it must be enlarged by balloon septostomy to ensure atrial mixing of the blood. An Arterial Switch Operation (ASO/Jetene Procedure) is routinely conducted to correct this defect. The ASO requires translocation of the great arteries and relocation of the coronary arteries. It is recommended that closure of VSDs occurs as early as possible after the atrial switch to prevent complications and improve survival rates. Without a surgical correction, infants with an intact ventricular septum typically do not survive past one year. Patients with VSDs or patent ductus arteriosus may survive beyond the first year, but almost always develop pulmonary vascular obstructive disease, limiting their long-term survival. The operation should be performed within the first few weeks of life when the ventricular septum is intact. A later time point is possible in cases of a hemodynamically significant VSD.
The ASO operative procedure can be divided into six stages. A median sternotomy or left thoracotomy will be performed to access the heart, followed by (1) pericardial harvest for future neopulmonary artery reconstruction. (2) Cannulation and cardiopulmonary bypass are applied due to standard methods for neonates with this specific defect. (3) Myocardial protection is achieved utilizing cardioplegia and iced saline slush during cardioplegic arrest. The next step is the (4) neoaortic reconstruction and coronary artery transfer. The coronary arteries are excised from their origin together with a surrounding button or arterial wall. This transfer is considered a critical feature of the operation. The great vessels are transected and the maneuver of Lecompte, which means translocating the pulmonary artery in front of the reconstructed aorta, can be performed. (5) Neopulmonary artery reconstruction aims to avoid the development of supravalvular stenosis and is achieved by pericardial patch augmentation. The final step is the (6) separation from cardiopulmonary bypass and postoperative care.
|


