|
Coarctication of the Aorta
Coarctation of the aorta is the eighth most common congenital heart defect and refers to a lesion whereby blood flow through the descending aorta is obstructed.
Anatomical description and types
The location of obstruction is variable, although is most commonly found at the insertion of the ductus arteriosus (Keane). This defect occurs frequently with a bicuspid aortic valve. Historically, coarctation has been classified as being either pre- or post-ductal, however this nomenclature is anatomically incorrect and is of little value in planning corrective surgery. A classification system based on the degree of aortic hypoplasia and associated lesions was developed to address this (St. Louis):
- Type I defects—involve narrowing in a segment of aorta adjacent to the ductus arteriosus insertion point
- Type II defects—narrowing in the same area as in Type I, in combination with hypoplasia of the aorta in the area between the ductus arteriosus and left subclavian artery.
- Type III defects—the most extreme form and involve a severe hypoplasia of a large portion of the aortic arch, typically between the left carotid artery and left subclavian artery.
Coarctation of the aorta is also commonly associated with other congenital defects such as VSDs, mitral valve abnormalities, complex heart disease, and an interrupted aortic arch. In the normal situation, the aortic arch narrows just superior to the insertion of the ductus arteriosus.
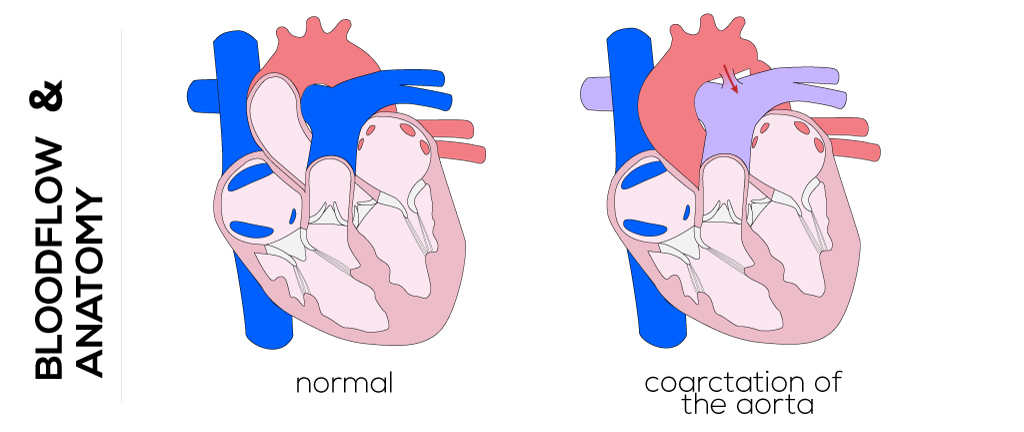
(Click picture to show/hide bloodflows)
Pathophysiology
Approximately, half of the patients with coarctation are asymptomatic or symptoms are overshadowed by other severe cardiac abnormalities. In the fetus, blood supply below this narrowing is supplied by the right ventricle via the patent ductus arteriosus while the blood above is supplied by the left ventricle via the ascending aorta. Symptoms and physiological deviations resulting from coarctation are due to left ventricular outflow tract obstruction and proximal systemic hypertension due to an increased afterload. It may cause differential blood flow to regions of the body, specifically upper and lower body. It typically becomes more obstructive as the child grows, but due to concurrent development of extensive collateral blood flow to circumvent the obstruction, differences between blood pressure in the arms and legs may not be apparent. In addition to variations in blood pressure between the upper and lower body, coarctation also causes hypertension and hypertrophy in the left ventricle.
Therapy
Management of this lesion depends on location and presence of associated cardiac anomalies. Early surgery is recommended for patients with uncomplicated coarctation and variation in systolic pressure between arms and legs of 20 mmHg. Medicinal therapy with diuretics and digitoxin helps to stabilize patients before surgical correction. The administration of prostaglandin E1 aims to maintain a patent ductus arteriosus. Mechanical ventilation and inotropic support are provided in the sickest infants.
The initial approach to coarctation utilizing open-heart surgery techniques was resection with primary end-to-end anastomosis, first reported in 1945, which was technically difficult and carried a high risk of re-intervention. This procedure was followed by a prosthetic patch aortoplasty, which showed later aneurysm development, prosthetic interposition grafts, and subclavian flap aortoplasty. The latter gained wide acceptance, but occurred with restenosis. Recently, an extended end-to-end anastomosis has become a popular surgical approach. In this process the patent ductus arteriosus is divided and its aortic end is removed with the constricted section of the aorta. The pulmonary end is ligated, whereas the ductal remnant is used to anastomose both ends of the aorta on either side of the coarctation.
Another technique developed in the last few years, but avoided in neonates, is percutaneous balloon angioplasty and transcatheter stent placement, also utilized for restenosis after surgical repair.
|


