|
Tricuspid Atresia
Anatomical description and types
Tricuspid atresia refers to a defect where the tricuspid valve is absent and the right ventricle is variably hypoplastic. An ASD is always present and can be restrictive. It can be classified into three types which have implications for clinical manifestations and surgical correction (St. Louis): 1) Type I includes those without transposition of the great arteries, 2) Type II includes those with transposition of the great arteries, and 3) Type III refers to those with congenitally corrected transposition of the great vessels. Each of these types can be subdivided based on the degree of obstruction to pulmonary blood flow:
- A—pulmonary arteries are atretic
- B—pulmonary stenosis or hypoplasia
- C—no obstruction to pulmonary flow
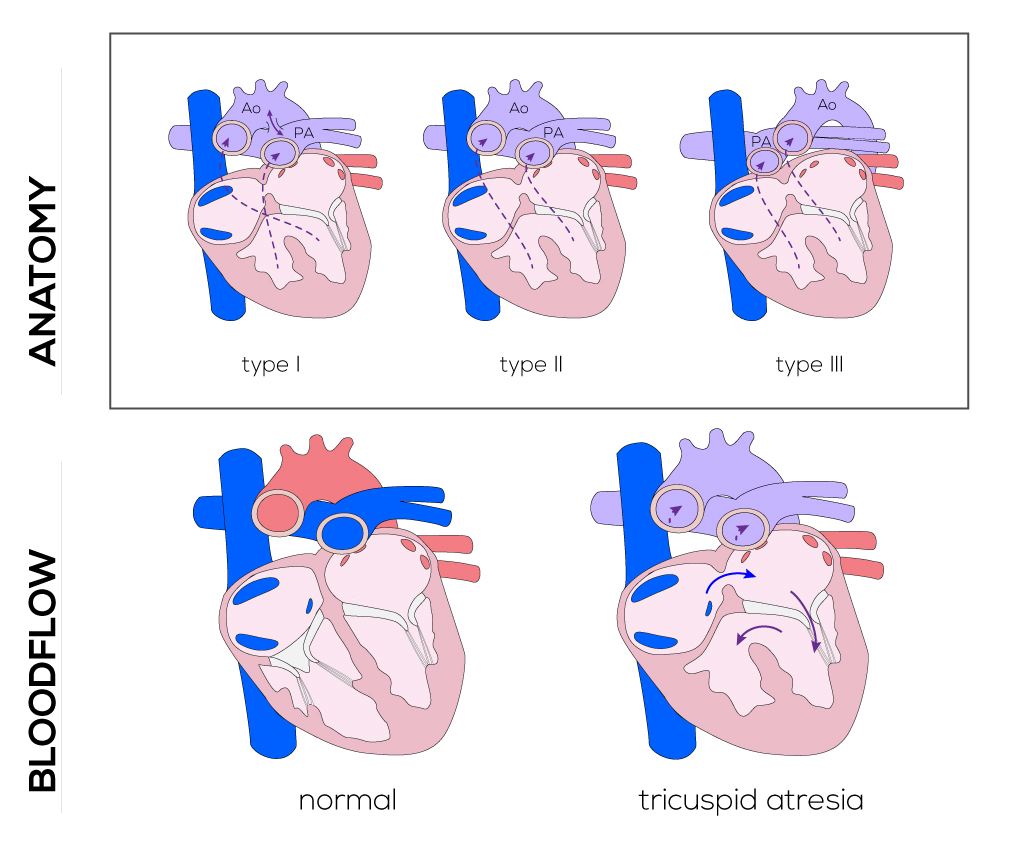
(Click picture to show/hide bloodflows)
Pathophysiology
When the tricuspid valve is absent, blood from the right atrium usually passes through a patent foramen ovale to the left atrium and left ventricle. In more rare cases, it may also pass through an ASD. Atresia of the tricuspid valve results in both the pulmonary and systemic venous returns passing through the foramen ovale and into the left atrium and left ventricle which functions as a single ventricle, mixing systemic and pulmonary venous return.
When the great vessels are normally related (Type I), blood passes from the left ventricle out the aorta and through a VSD to the right ventricle and pulmonary artery. Closure of the septal defect, combined with restriction by the pulmonary valve and limited right ventricular outflow due to hypoplasia, results in a progressive decrease in pulmonary blood flow. The patent ductus arteriosus provides a time-limited source of blood to the pulmonary artery, as it typically closes on schedule a few days after birth.
When the great vessels are transposed (Type II), blood from the left ventricle passes out the pulmonary artery and through a VSD into the aorta. Closure of the septal defect leads to subaortic stenosis and a decrease in cardiac output. To compensate, pressure in the left ventricle increases which, in turn, increases pulmonary outflow leading to congestive heart failure.
Most patients with tricuspid atresia present with cyanosis that increases in severity over a period of months as VSDs and the ductus arteriosus constrict. As the degree of cyanosis worsens, infants may also begin to have cyanotic spells.
Therapy
In neonates with a severe restrictive VSD or pulmonary atresia, a prostaglandin E1 infusion is administered to keep the ductus arteriosus open. Surgical management of tricuspid atresia typically involves 3 stages of operations to eventually accomplish a modified Fontan procedure around 2 to 4 years of age.
The first stage of the neonatal palliative procedure is performed to insure pulmonary blood flow, often accomplished by a modified Blalock-Taussing shunt and can be omitted when pulmonary arterial pressure is appropriate. This shunt is constructed by interposing a graft between the subclavian artery and the right or left pulmonary artery. Originally performed through a right/left thoracotomy depending on the neonate’s anatomy, the procedure is nowadays usually completed through a median sternotomy. It allows for a shorter shunt which can be placed more centrally. By the time of definitive repair, a re-sternotomy is necessary.
The second stage is the bi-directional Glenn or Hemi-Fontan procedure performed on children between 4 and 9 months of life. The Glenn procedure involves a superior cavopulmonary anastomosis as a first step to separate the systemic and pulmonary circulations, which reduces the volume work of the ‘single ventricle.’ If present, the Blalock-Taussing shunt will be clamped and disconnected and other additional sources of pulmonary blood flow as well. The divided superior vena cava is connected to the right pulmonary artery which will stay connected to the left pulmonary artery. Consequently, deoxygenated blood from the head and neck flows directly into the pulmonary artery. The azygos vein is usually ligated too. The Hemi-Fontan is likewise a cavopulmonary anastomosis which is intended to simplify the eventual completion of the Fontan circulation. The superior vena cava is connected to the pulmonary artery while the interface between the superior vena cava and the right atrium is closed by a patch. An additional patch is used to endorse the merge of the superior vena cava and, with the pulmonary arteries, to address possible stenosis or distortion. When a lateral tunnel (versus an extra-cardiac conduit) is planned to accomplish total completion of the cavopulmonary circulation, the latter procedure is preferred.
Afterwards, the third stage and actual Fontan procedure will be performed. A pathway or tunnel either inside or outside of the heart (lateral atrial tunnel or extra-cardiac conduit) is constructed to connect the inferior vena cava with the pulmonary artery. A small opening in the venous pathway is created to compensate for high right-sided pressures. Nearly normal oxygen saturation will be achieved after this procedure.
|


