|
Heart/Lung Machine (1951)
Prior to 1950, the heart was considered to be the core of human emotion, even the soul itself. When the medical profession eventually began to view the heart more physiologically, as a pump or machine within the body, researchers and clinicians began to develop new ways to repair and replace worn-out parts of the heart. Innovations in the field of cardiac surgery then flourished at the University of Minnesota. For example, Dr. Clarence Dennis designed one of the first heart-lung machines for total cardiopulmonary bypass which was subsequently tested successfully on dogs, albeit with less success on humans.
Hypothermia (1952)
Worldwide, one of the next major milestones in cardiac surgery was the first open-heart surgery performed using hypothermia, a procedure first attempted in 1952 by Drs. F. John Lewis, Richard Varco, and colleagues at the University of Minnesota. This procedure lowered the body temperature of patients 12-15°F to reduce their blood flow, thereby reducing the body's need for oxygen. Brain cells would die after 3-4 minutes at normal temperature without oxygen, but hypothermia allowed the University of Minnesota surgical team (Drs. F. John Lewis, C. Walton Lillehei, Mansur Taufic, and Richard Varco) to successfully complete a 5 1/2-minute repair of the atrial septum of a five-year-old patient. This was recognized as a significant landmark in the history of cardiac surgery; until this time, no surgeon had succeeded in opening the heart to perform intracardiac repair under direct vision.
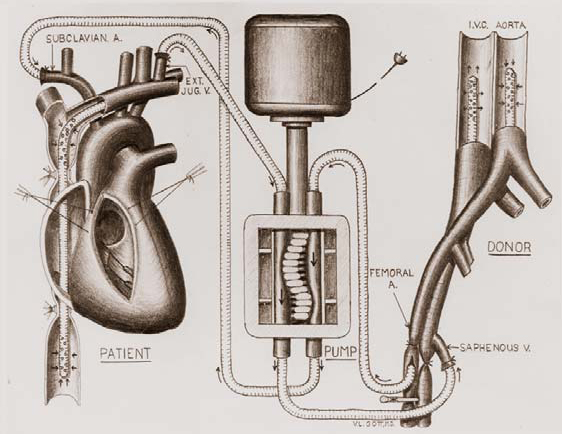
Figure 4. Diagram of cross-circulation.
Cross-circulation (1954)
Extracorporeal circulation by controlled cross-circulation was introduced clinically in 1954, after rigorous animal experimentation (Figure 4). The use of cross-circulation for intracardiac operations was an immense departure from established surgical practice at the time, and was considered as a major breakthrough that motivated numerous innovations in the area of open-heart surgery [3]. The thought of taking a normal healthy human being into the operating room to provide donor circulation was considered unacceptable and even immoral by some critics; the risks to the donors included blood incompatibility, infection, air embolism (stroke), and/or blood volume imbalances.
From March 1955 onward, three additional bypass methods were introduced and successfully used, including: 1) perfusion from a reservoir of arterialized blood; 2) heterologous (dog) lungs as an oxygenator; and 3) the DeWall-Lillehei disposable bubble oxygenator [4]. Yet, many believe that the single most important discovery that contributed to the success of clinical open-heart operations was the realization of the vast discrepancy between the total body flow rate thought necessary and what was actually necessary to maintain cerebral viability. Lillehei and his team are credited with applying the findings of two British surgeons (Andreasen and Watson) who identified the azygos factor - the ability of dogs to survive up to 40 minutes without brain damage when all blood flow was stopped except through the azygos vein. Specifically, Morley Cohen and Lillehei hypothesized that when blood flow was low, the blood vessels dilated to receive a larger share of the blood, while the tissues absorbed a much higher proportion of the oxygen as compared to normal circulation [4]. Previously, it was thought that basal or resting cardiac output at 100-160 ml/kg/min was safe maintenance during cardiopulmonary bypass. The azygos flow studies showed that 8-14 ml/kg/min maintained the physiological integrity of the vital centers, but Lillehei added a margin of safety and set his basic perfusion rate at 25-30 ml/kg/min. This approach reduced excessive complications of blood loss, excessive hemolysis, abnormal bleeding, and/or renal shutdown [4].
Altogether, forty-five patients (aged 5 months to 10 years) underwent open-heart surgery with the cross-circulation approach at the University in 1954-1955; prior to these pioneering surgeries, such patients were considered to have lesions that were hopelessly unrepairable. Of this group, twenty-two (49%) of the patients lived to be long-term survivors (greater than 30 years) and lead normal productive lives; eleven of the female long-term survivors subsequently gave birth to a total of 25 children who were free from any congenital heart defects. In addition, all forty-five donors survived, with only one donor experiencing a major complication. At a more recent 53-year follow-up, twenty (44%) of the original cross-circulation patients were living with no problems or significant limitations related to their original surgeries [5]. Although its clinical use was short-lived, cross-circulation is still considered today as one of the most important stepping stones in the development of the discipline of cardiac surgery.

Figure 5. University of Minnesota's bubble oxygenator cost $15 and was easy to use. Richard DeWall is shown here with his model in 1955.
Lillehei-DeWall Bubble Oxygenator (1955)
Importantly, John Gibbon, MD, from Boston, invented the cardiopulmonary bypass procedure and performed the first intracardiac repair using extracorporeal perfusion in 1953. His bubble oxygenator, which looked surprisingly like a computer, was manufactured and financed by IBM. His reported achievements stimulated rapid development of the knowledge base and equipment necessary for both accurate diagnoses of cardiac disease and successful intracardiac operations. Yet at that time, it was recognized that the main problems with film oxygenators were: 1) poor efficiency; 2) excessive hemolysis; 3) large priming volumes; and 4) development of bubbles and foam in the blood. All designs required blood flows of 2.2 l/m2 /min, usually three to four units of blood for priming, and another two units for the remainder of the circuit. Furthermore, after each use, the machine needed to be broken down, washed, rinsed in hemolytic solution, reassembled, resterilized, and reconfigured.
During this era, Richard DeWall worked at the University of Minnesota as an animal attendant in Lillehei's research laboratory. It was noted that DeWall would manage the pump while the anesthesiologists would take breaks, and soon he began to take an interest in the problems associated with oxygenating blood. Eventually, Lillehei challenged DeWall to find a way to eliminate bubbles in the oxygenator procedure. Importantly, DeWall brought to fruition a dramatic technological breakthrough in 1955 by developing the first bubble oxygenator with a unique method for removing bubbles from the freshly oxygenated blood (Figure 5). In DeWall's design, blood entered the bottom of a tall cylinder along with oxygen passed through sintered glass to create bubbles. As the bubbles and blood rose, gas exchange occurred at the surface of each bubble. At the top of the cylinder, arterialized bubble-rich blood passed over stainless steel wool coated with silicone antifoam; it then traveled through a long helical settling coil to allow bubbles to slowly rise and exit the blood. Two important components in the Lillehei-DeWall bubble oxygenator were the tubing and the silicon antifoam solution. The tubing was Mayon polyethylene tubing (typically used in the dairy and beer industries and specifically in the production of mayonnaise), and the silicone antifoam solution, Antifoam A, was used to coat the tubing to prevent foaming of the liquids being transported.
The oxygenator was wonderfully efficient; experimental animals (and later patients) did not show detectable effects of residual gas emboli. More importantly, this design eventually led to the development of a plastic, prepackaged, disposable, sterile oxygenator that replaced the expensive stainless steel, labor-intensive screen and film devices. An economic and reliable oxygenator had arrived and the medical industry began to consider using disposable components for the heart-lung machine.
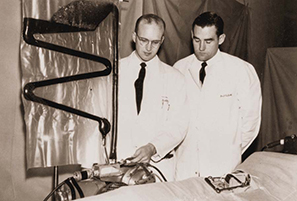
Figure 6. Richard DeWall and Vincent Gott look at the first commercially manufactured sterile bubble oxygenator in 1956.
Two years after its introduction, the DeWall-Lillehei bubble oxygenator had been used in 350 open-heart operations at the University of Minnesota. DeWall steadily improved the device through three models, but it remained a very simple, disposable, heat-sterilizable device that could be built to accommodate only the amount of blood required for each patient and then discarded.
In 1956, another one of Lillehei's residents, Vincent Gott, invented a bubble oxygenator in which DeWall's helix design was flattened and enclosed between two heat-sealed plastic sheets (Figure 6). This sheet bubble oxygenator proved to be the key to widespread acceptance of the device in open-heart surgery, because it could be easily manufactured and distributed in a sterile package, and it was inexpensive enough to be disposable. With the bubble oxygenator and techniques developed by Lillehei and his colleagues, the University of Minnesota had become even more prominent for making open-heart surgery possible and relatively safe [6].
|


