















|
 |
|
|
|
|
Cardiac Resynchronization Therapy
In the past decade, the coronary venous system has also been used as a pacing lead implant site for Cardiac Resynchronization Therapy (CRT). CRT involves pacing at least two ventricular sites in order to minimize the required time for total ventricular activation and thus improve cardiac synchrony in certain patients with heart failure eliciting ventricular dyssynchrony [1-2]. Implantation of pacing leads via the coronary venous system is currently the most popular approach for left ventricular pacing and is accomplished by a transvenous approach [3]. Several studies have reported the beneficial results of pacing from the lateral or inferolateral region of the left ventricle [3-8]; either the lateral vein or inferior vein of the left ventricle are considered optimal implant sites [1,9-10]. However, it should be noted that, to date, response rates to CRT are still suboptimal with a typical success rate of around two-thirds [11-13].
Conduit for Therapy
Because the coronary venous system is not prone to the effects of atherosclerotic disease, it is considered that it may also serve as an effective conduit for drug delivery or as a potential avenue for coronary artery bypass. For example, for decades, the distribution of cardioplegia through the coronary sinus has been proven to be safe and effective in myocardial protection, and even superior to the traditional method of antegrade cardioplegia, especially in patients with coronary artery disease [14]. More recently, since restoration of coronary blood flow prior to an acute myocardial infarction can significantly reduce infarct size and improve myocardial function, the administration of recombinant tissue-type plasminogen through the coronary venous system was shown to result in both shorter recovery times and significant reduction in infarct size when compared to intravenous administration [15]. The coronary venous system also can be employed to deliver cell therapy directly to the myocardium as a potential treatment for heart failure [16]. In one case study, it was demonstrated that a catheter-based system allowed arterialization of a cardiac vein to bypass a totally occluded left anterior descending coronary artery [13,17].
|
|
|
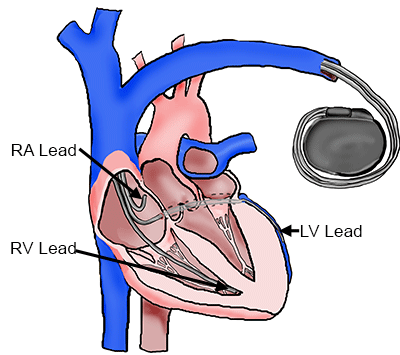
Cardiac Resynchronization Therapy System
|
|
|
Other Biomedical Applications
The coronary sinus and great cardiac vein have specifically been used for a number of cardiac therapies. For example, the coronary sinus can be used as a means to deliver ablation therapy [18-21]. An ablation catheter can be placed within the coronary sinus to treat atrial fibrillation in the left atrium. Also, a reducer stent can be deployed within the coronary sinus to relieve chronic angina symptoms [22]. The reducer stent decreases the diameter of the vein to increase pressure in the coronary arteries, which then can increase blood flow to ischemic areas of the heart. The increase in blood flow can reduce angina. Finally, mitral valve annuloplasty devices can also be implanted in the coronary sinus and great cardiac vein surrounding the mitral valve annulus to reduce mitral regurgitation [23-24].
References
- Barold S (2001) What is cardiac resynchronization therapy? Am J Med 111:224-232
- Casey C, Knight B (2004) Cardiac resynchronization pacing therapy. Cardiology 101:72-78
- Daubert J, Ritter P, Le Breton H et al (1998) Permanent left ventricular pacing with transvenous leads inserted into the coronary veins. Pacing Clin Electrophysiol 21:239-245
- Walker S, Levy T, Rex D et al (2000) Initial United Kingdom experience with the use of permanent, biventricular pacemakers, Implantation procedure and technical considerations. Europace 2:233-239
- Gasparini M, Mantice M, Galimberti P et al (2003) Is the left ventricular lateral wall the best lead implantation site for cardiac resynchronization therapy? Pacing Clin Electrophysiol 26:162-168
- Stevenson W, Sweeney M (2004) Single site left ventricular pacing for cardiac resynchronization. Circulation 109:1694-1696
- Valls-Bertault V, Mansourati J, Gilard M, Etienne Y et al (2001) Adverse events with transvenous left ventricular pacing in patients with severe heart failure, Early experience from a single centre. Europace 3:60-63
- Abraham W et al (2004) Effects of cardiac resynchronization on disease progression in patients with left ventricular systolic dysfunction, an indication for an implantable cardioverter-defibrillator and mildly symptomatic chronic heart failure. Circulation 110(18):2864-2868
- Alonso C, Leclercq C, d'Allonnes F et al (2001) Six year experience of transvenous left ventricular lead implantation for permanent biventricular pacing in patients with advanced heart failure, technical aspects. Heart 86:405-410
- Rossillo A et al (2004) Impact of coronary sinus lead position on biventricular pacing, mortality and echocardiographic evaluation during long-term follow-up. J cardiovasc electrophysiol 15(10):1120-1125
- Conti C (2006) Cardiac resynchronization therapy for chronic heart failure, Why does it not always work? Clin Cardiol 29:335-336
- Yu C, Wing-Hong F, Zhang Q, Sanderson J (2005) Understanding nonresponders of cardiac resynchronization therapy--current and future perspectives. J Cardiovasc Electrophysiol 16:1117-1124
- Anderson S, Lahm R, Iaizzo, P (2009) The Coronary Vascular System and Associated Medical Devices. Handbook of Cardiac Anatomy, Physiology , and Devices. Springer, New York: 109-123
- Gundry S (1982) A comparison of retrograde cardioplegia versus antegrade cardioplegia in the presence of coronary artery obstruction. In: Scientific Session of the American Heart Association, Dallas
- Miyazaki A, Tadokoro H, Drury J et al (1991) Retrograde coronary venous administration of recombinant tissue-type plasminogen activator, a unique and effective approach to coronary artery thrombolysis. J Am Coll Cardiol 18:613-620
- Thompson C, Nasseri B, Makower J et al (2003) Percutaneous transvenous cellular cardiomyoplasty: A novel nonsurgical approach for myocardial cell transplantation. J Am Coll Cardiol 41:1964-1971
- Oesterle S, Reifart N, Hauptmann E et al (2001) Percutaneous in situ coronary venous arterialization, report of the first human catheter-based coronary artery bypass, Circulation 103:2539-2543
- Fisher J et al (1984) Attempted nonsurgical electrical ablation of accessory pathways via the coronary sinus in the Wolff-Parkinson-White syndrome. J Am Coll Cardiol 4:685-694
- Haissaguerre M et al (1992) Radiofrequency catheter ablation of left lateral accessory pathways via the coronary sinus. Circulation 86(5):1464-1468
- Gaita F, Riccard P, Ferraro A (2002) Cryothermic ablation within the coronary sinus of an epicardial posterolateral pathway. J Cardio Electro 13(11):1160-1163
- Haissaguerre M et al (2007) Impact of catheter ablation of the coronary sinus on paroxysmal or persistent atrial fibrillation. J cardiovasc electrophysiol 18(4):378-386
- Banai S et al (2007) Coronary sinus reducer stent for the treatment of chronic refractory angina pectoris, a prospective, open label, multicenter, safety feasibility first-in-man study. J Am Coll Cardiol 49(17):1783-1789
- Harnek J et al (2011) Transcatheter implantation of the MONARC coronary sinus device for mitral regurgitation, 1 year results from the EVOLUTION phase I study JACC 4(1):115-122
- Choure A et al (2006) In vivo analysis of the anatomical relationship of coronary sinus to mitral annulus and left circumflex coronary artery using cardiac multidetector computed tomography. J Am Coll Cardiol 15(7):1938-1945
|
|
|
 |
|


