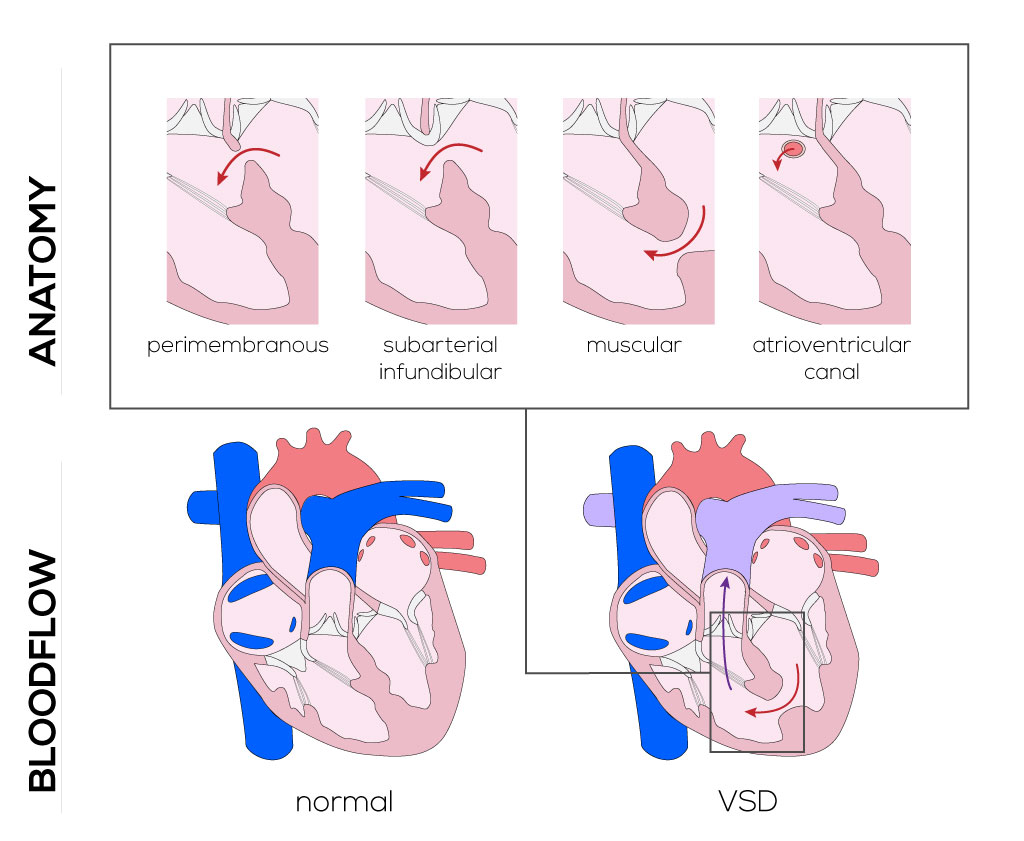
- Subarterial infundibular type defect—located in the areas normally formed by the infundibular septum with the non-muscular rim formed by the adjacent arterial valves in the right ventricle outflow tract. The defect lies caudal from the pulmonary valve. The superior rim might have a relationship with the right coronary cusp of the aortic valve.
- Muscular type defect—may exist in any one of three areas. Inlet muscular defects are found in the areas between the trabecular and inlet septa. Infundibular muscular defects are located between the infundibular and trabecular septa, and trabecular muscular defects originate in the infundibulum itself. These defects are difficult to visualize and repair, because they often occur as multiple holes with various shapes and borders on different planes.
- Atrioventricular canal or inlet type defect—located posteriorly, immediately subjacent to the tricuspid valve septal leaflet in the inlet portion of the ventricular septum. These defects result from embryologic development evolving the endocardial cushion. The conduction system is at risk during surgical reparation due to its anatomical proximity to the atrioventricular node and conduction bundles that traverse along the inferior margin of the defect.
(Click picture to show/hide bloodflows)
Pathophysiology
VSDs allow for left-to-right shunting, beginning at birth when pulmonary resistance falls and systemic pressures increase. Additional blood passing through the right ventricles, pulmonary arteries, and left atrium may lead to congestive heart failure as well as hypertension of all three structures. Clinical manifestations vary depending on the size of the defects and the degree of shunting that occurs. Clinical manifestations may develop within days after birth or take up to 6 months, depending on severity of the defects, presence of additional cardiac defects, and/or elicitation of other extracardiac anomalies. Most infants are asymptomatic, as defects are often too small to allow for significant left-to-right shunting. Larger defects can manifest themselves as congestive heart failure, respiratory infections, tachypnea, dyspnea, and/or altered growth rates. Patients with restrictive VSDs are diagnosed by holosystolic murmur and two-dimensional echocardiography coupled with Doppler flow techniques.
Therapy
The management of a VSD is dependent on its size and location. In general, the majority of defects are small and can typically be managed conservatively, i.e., if the child reaches 6 months of age without evidence of pulmonary hypertension. VSDs do not enlarge with age, rather some are known to decrease in size and occasionally close spontaneously. Pharmacological treatment such as digitalis, diuretic therapy, and antibiotic therapy often delay surgical treatment. Still, it is commonly recommended that larger defects be surgically closed within the first months of life.
The method of the defect closure utilizing patches depends on the patient’s history, size, type of VSD, intervening symptoms, and complicating factors. In general, there are five operative approaches for closure: 1) right atrial (most common), 2) transpulmonary, 3) transaortic, 4) right ventricular, and 5) left ventricular. In the case of multiple defects, a combined approach is necessary. To protect the myocardium and provide a relatively bloodless surgical field, aortic cross-clamping, aortobicaval cannulation, cardioplegia, hypothermia, and left ventricular vent are applied.
The right atrial approach is applicable to paramembranous, inlet, and muscular defects. After opening of the right atrium, the tricuspid valve leaflets need to be retracted in order to expose the defect. Injuries of the conduction system, the aortic valve, and the tricuspid valve leaflets can be avoided by placing sutures superficially along the inferior and posterior margin of the defect to secure the patch.
The transpulmonary approach is usually used for conal (supracristal) defects. The use of a patch is particularly important, because the patch helps support the prolapsing aortic valve. It is important to visualize the size of the defect, which could be hidden by the prolapsing aorta, and use proper sized patches to cover the full extent of the hole. The patch should not interfere with the pulmonic valve function, yet it needs to support the previously prolapsed aortic valve.
Closure of a VSD via an aortic incision, the transaortic approach, is usually performed when other lesions need to be repaired concomitantly such as aortic valvuloplasty for prolapsed valve or relief of valvar/subvalvar stenosis. The obliquely curved incision is made from the anterior ascending aorta above the aorta valve commissure to a level above the center of the right coronary sinus. To expose the defect, aortic valve leaflets are retracted and a patch can be secured utilizing different suture methods and an appropriate patch.
The right ventricular approach is infrequently used, likely when none of the previously explained methods can be applied. This is the case when there is a paramembranous defect extending superiorly to the infundibular septum among other rather rare circumstances. Either a transverse or vertical incision is made starting in the middle of the anterior infundibular wall. Examination of the coronary artery distribution is required to avoid injuries.
The left ventricular approach is limited to certain types of defects, such as multiple apical, sieve-like perforations (swiss cheese). The more commonly used incision is vertical (versus transverse) starting in the relatively avascular left ventricular apical area with limited extension. Long-term ventricular dysfunction may result however, therefore it is best to avoid this method whenever possible.
It should be noted that knowledge and awareness of the conduction system during these surgical procedures is essential. The atrioventricular node as well as right and left bundles may be damaged by a suturing needle, from snaring by suture material, by fibrosis from hemorrhage, or anoxic insult. Also direct pressure trauma from rough handling of surgical instruments may cause disturbances to the conduction system.
As an alternate to the surgical approach, transcatheter closure devices became a reliable method in the last decade with acceptable mortality and morbidity rates, as well as encouraging results on selected patients. Two available devices for this are the STARFlex and Amplatzer muscular ventricular septal occluder. For device delivery, a catheter is usually introduced into the left ventricle and an arterial-venous loop is then formed. After deploying a delivery sheath from the venous side, the device be carefully placed with transesophageal guidance.
Synopsis
- Membranous defects tend to improve with time and may be managed conservatively, i.e., if pulmonary arterial pressures remain low.
- Muscular defects may also close spontaneously, particularly if the lesions are small. Yet, if significant shunting and pulmonary hypertension occur, it is recommended that these defects be closed (either surgically or in a minimally invasive manner).
- Defects in the infundibular region are typically large and, unlike those in other areas of the septum, do not decrease in size. For these reasons, it is recommended that infundibular lesions be surgically corrected.
- Atrioventricular canal defects almost always require surgical intervention because, like the infundibular types, they are typically large and do not spontaneously decrease in size.
|



