|
In order to sustain viability, it is not possible for nutrients to diffuse from the chambers of
the heart through all the layers of cells that make up the heart tissue. Thus, the coronary
circulation is responsible for delivering blood to the heart tissue itself (the myocardium). The
normal heart functions almost exclusively as an aerobic organ with little capacity for anaerobic
metabolism to produce energy. Even during resting conditions, 70 to 80% of the oxygen available
within the blood circulating through the coronary vessels is extracted by the myocardium. It then
follows that because of the limited ability of the heart to increase oxygen availability by further
increasing oxygen extraction, increases in myocardial demand for oxygen (e.g., during exercise or
stress) must be met by equivalent increases in coronary blood flow. Myocardial ischemia results
when the arterial blood supply fails to meet the needs of the heart muscle, for oxygen and/or
metabolic substrates. Even mild cardiac ischemia can result in anginal pain, electrical changes
(detected on an electrocardiogram) and the cessation of regional cardiac contractile function.
Sustained ischemia within a given myocardial region will most likely result in an infarction.
As noted above, as in any microcirculatory bed, the greatest resistance to coronary blood flow
occurs in the arterioles. Blood flow through such vessels varies approximately with the fourth
power of these vessels' radii; hence, the key regulated variable for the control of coronary blood
flow is the degree of constriction or dilatation of coronary arteriolar vascular smooth muscle. As
with all systemic vascular beds, the degree of coronary arteriolar smooth muscle tone is normally
controlled by multiple independent negative feedback loops. These mechanisms include various
neural, hormonal, local non-metabolic and local metabolic regulators. It should be noted that the
local metabolic regulators of arteriolar tone are usually the most important for coronary flow
regulation; these feedback systems involve oxygen demands of the local cardiac myocytes. In
general, at any one point in time, coronary blood flow is determined by integrating all the
different controlling feedback loops into a single response (i.e., inducing either arteriolar smooth
muscle constriction or dilation). It is also common to consider that some of these feedback loops
are in opposition to one another. Interestingly, coronary arteriolar vasodilation from a resting
state to one of intense exercise can result in an increase of mean coronary blood flow from
approximately 0.5 to 4.0 ml/min/gram.
As with all systemic circulatory vascular beds, the aortic or arterial pressure (perfusion
pressure) is vital for driving blood through the coronaries, and thus needs to be considered as
another important determinant of coronary flow. More specifically, coronary blood flow varies
directly with the pressure across the coronary microcirculation, which can be essentially considered
as the aortic pressure, since coronary venous pressure is near zero. However, since the coronary
circulation perfuses the heart, some very unique determinants for flow through these capillary beds
may also occur; during systole, myocardial extravascular compression causes coronary flow to be near
zero, yet it is relatively high during diastole (note that this is the opposite of all other
vascular beds in the body).
|
Oxygenated blood is pumped into the aorta from the left ventricle. This is where it enters the
right and left main coronary arteries, and subsequent branching feeds the myocardial tissue of all
four chambers of the heart (see Figure 7). The ascending portion of the aorta is where the origins
(ostia) of the right and left coronaries reside; specifically, they exit the ascending aorta
immediately superior to the aortic valve at the sinus of Valsalva. Blood flow into the coronary
arteries is greatest during ventricular diastole when aortic pressure is highest and it is greater
than in the coronaries. Typically the right coronary artery courses along the right anterior
atrioventricular groove just below the right atrial appendage and along the epicardial surface
adjacent to the tricuspid valve annulus. It traverses along the tricuspid annulus until it reaches
the posterior surface of the heart, where it then commonly becomes the posterior descending artery
and runs toward the apex of the left ventricle. Along its course, a number of branches emerge, most
notably those that supply the sinus node and the atrioventricular node; hence blockage of such
vessels can lead to conduction abnormalities. Additionally, several marginal branches run to the
right ventricular and right atrial epicardial surfaces. The left main coronary artery typically
bifurcates quickly upon exiting the ascending aorta into the left circumflex and left anterior
descending arteries. The left circumflex artery runs under the left atrial appendage on its way to
the lateral wall of the left ventricle. Along the way, it spawns a number of branches that supply
the left atrial and left ventricular walls. In some cases, a branch will course behind the aorta to
the superior vena cava such that it can supply the sinus node. The left anterior descending artery
supplies a major portion of the ventricular septum, including the right and left bundle branches of
the myocardial conduction system, and the anterior and apical portions of the left ventricle.
|
|
|
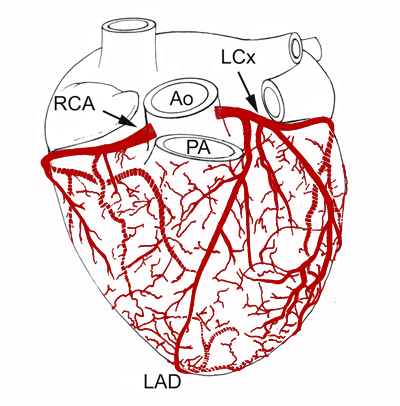
Figure 7. Drawing of the coronary arterial circulation in the human heart. The normal human
hears does not typically elicit collateralization; each area of myocardium is usually supplied by a
single coronary artery. Ao = aorta; LAD = left anterior descending artery; LCx = left circumflex
artery; PA = pulmonary artery; RCA = right coronary artery.
|
|
Coronary arteries are so vital to the function of heart; whenever disease states are associated
with flow restriction through the coronary arteries, and subsequently the remainder of the coronary
circulations (capillaries and veins), the effects on cardiac performance are quite dramatic and
often fatal. Coronary artery disease (CAD) is generally defined as the gradual narrowing of the
lumen of the coronary arteries due to coronary atherosclerosis. Atherosclerosis is a condition that
involves thickening of the arterial walls from cholesterol and fat deposits that build up along the
endoluminal surface of the arteries. With severe disease, these plaques may become calcified and so
large that they produce stenoses within the vessels, and thus permanently increase the vascular
resistance which is normally low. When the walls of the coronary arteries thicken, the
cross-sectional area of the arterial lumen decreases; resulting in higher resistance to blood flow
through the coronary arteries. This steady decrease in cross-sectional area can eventually lead to
complete blockage of the artery. As a result, oxygen and nutrient supply to the myocardium drops
below the demand of the myocardium. As the disease progresses, the myocardium downstream from the
occluded artery becomes ischemic. Eventually, myocardial infarction (or known as a MI) may occur if
the coronary artery disease is not detected and treated in a timely manner.
Myocardial ischemia not only impairs the electrical and mechanical function of the heart, but
also commonly results in intense, debilitating chest pain known as angina pectoris. However,
anginal pain can often be absent in individuals with coronary artery disease when they are resting
(or in individuals with early disease stages), but induced during physical exertion or with
emotional excitement.
|


