|
Hypoplastic Left Heart Syndrome
Anatomical description and types
Hypoplastic left heart syndrome describes a defect where the left ventricle is too small to support systemic circulation. It is characterized by hypoplasia of the left ventricle, hypoplasia or atresia of the mitral valve, and hypoplasia of the ascending aorta. The aorta can present with a decreased diameter and coarctation. Often an ASD is located superiorly on the septum.
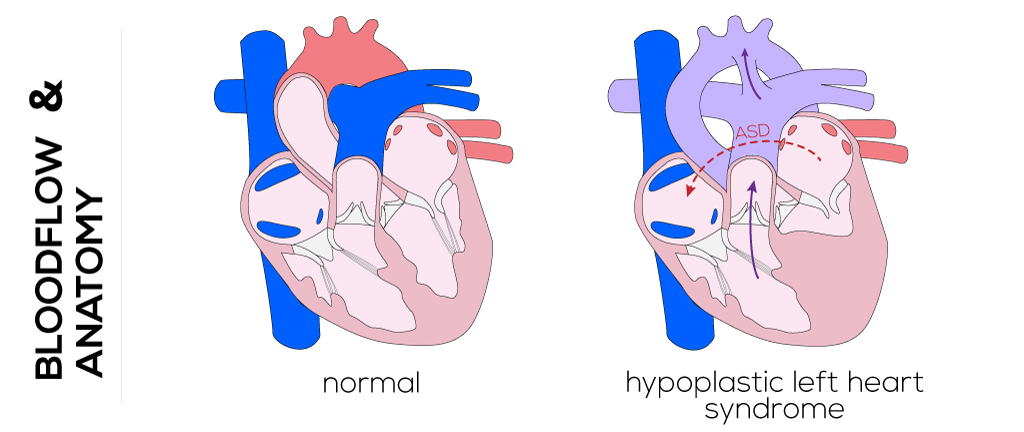
(Click picture to show/hide bloodflows)
Pathophysiology
In the absence of a fully functional left ventricle and/or aortic valve, preservation of systemic circulation and survival is dependent on a patent ductus arteriosus. In this case, the right ventricle takes over for the left to supply the pulmonary and systemic circuits as well as the coronary arteries and brachiocephalic veins (in a retrograde direction). The amount of blood flow to the pulmonary and systemic circulation is a function of the vascular resistance which, in turn, is influenced by the size of the interatrial orifice. Arterial defects that restrict movement cause pressure in the left atrium and pulmonary circuit to increase, and thus restrict pulmonary blood flow. Increased pulmonary blood flow brings a larger volume of oxygenated blood back to the left atrium, which then mixes with the systemic venous return in the right via the foramen ovale.
During the prenatal period all blood, aside from a small volume of pulmonary venous return, passes through the right heart. Return from the superior and inferior vena cavae, pulmonary venous system, and oxygenated blood from the placenta combine in the right atrium. The right ventricle receives this blood and, taking over for the left ventricle, distributes it to the body and coronary arteries making use of the connection between the ductus arteriosus and aorta.
The newborn with hypoplastic left heart syndrome may appear normal after birth while the ductus arteriosus is patent and pulmonary vascular resistance is relatively high. When the ductus closes, however, systemic circulation and perfusion of the coronary arteries become restricted and the infant may begin to appear cyanotic or pale with decreased peripheral pulses. The decrease in pulmonary resistance that occurs with expansion of the lungs allows pulmonary blood flow to increase and can cause tachypnea. Eventually, these infants develop cardiogenic shock, acidosis, and renal failure and they typically die quickly without intervention.
Therapy
Management of hypoplastic left heart syndrome begins with administration of prostaglandin E1 to maintain a patent ductus arteriosus and thus, pulmonary and systemic blood flow. The goal of pre-surgical interventions is to maintain systemic circulation, increase pulmonary vascular resistance, and reduce the right ventricular volume load. Sub-ambient oxygen delivery with nitrogen are life-sustaining after birth to maintain systemic perfusion.
Three goals have been established for initial successful palliation by surgical intervention: 1) establish a permanent unobstructed communication between the right ventricle and aorta while preserving right ventricular function, 2) limit pulmonary blood flow while preserving pulmonary artery architecture, and 3) relieve pulmonary venous obstruction. These operations require standard open-heart procedure techniques such as cardiopulmonary bypass, hypothermia, and cardiac arrest.
The first stage in surgical palliation shortly after birth is the Norwood Procedure or modification of the Norwood procedure. It aims to: 1) merge the proximal main pulmonary artery with the aorta to guarantee unobstructed aortic (systemic) blood flow, 2) insure pulmonary venous return to the systemic ventricle via an atrial septectomy, and (3) regulate/adjust pulmonary blood flow. The latter can be managed by applying a Blalock-Taussing shunt which guarantees adequate perfusion of the lungs and prevents overcirculation by lowering the pulmonary pressure in comparison to the systemic pressure. This shunt is constructed by interposing a graft between the subclavian artery and the right or left pulmonary artery. Originally performed through a right/left thoracotomy depending on the neonate’s anatomy, the procedure is usually completed through a median sternotomy. It allows for a shorter shunt which can be placed more centrally. Alternatively, a conduit can be placed between the systemic ventricle and the pulmonary artery (Sano modification).
The second stage is the bi-directional Glenn or Hemi-Fontan procedure performed on children in the following 2 to 10 months of life. The Glenn procedure is a superior cavopulmonary anastomosis used as a first step to separate the systemic and pulmonary circulations, which reduces the volume work of the ‘single ventricle.’ The Blalock-Taussing shunt will be disconnected along with other sources of pulmonary blood flow as well. The superior vena cava will be separated from the right atrium and connected to the right pulmonary artery. Consequently, deoxygenated blood from the head and neck will flow directly into the pulmonary artery. The azygos vein is usually ligated too. The Hemi-Fontan is likewise a cavopulmonary anastomosis which is intended to simplify the eventual completion of the Fontan circulation. The superior vena cava is connected to the pulmonary artery. The interface between the superior vena cava and the right atrium is closed by a patch. An additional patch is used to endorse the merge of the superior vena cava and with the pulmonary arteries and to address possible stenosis or distortion. When a lateral tunnel (versus an extra-cardiac conduit) is planned to accomplish total completion of the cavopulmonary circulation, the latter procedure is preferred.
Afterwards in the age of 18 to 24 months, the third stage and actual Fontan procedure will be performed. A pathway or tunnel either inside or outside of the heart (lateral atrial tunnel or extra-cardiac conduit) is constructed to connect the inferior vena cava with the pulmonary artery. A small opening in the venous pathway is created to compensate high right-sided pressures, and a nearly normal oxygen saturation will be achieved after this procedure.
Initial palliative surgery carries the risk of numerous potential complications that can be related to the anatomy of the defect itself or surgical manipulation. These must be monitored and may require additional surgeries. Cardiac transplantation is a treatment option that may be available depending on donor availability and probability of rejection.
|


