|
Overview of Cardiac Conduction
|
The sinoatrial node is located in the upper part of the right atrium in the healthy heart, and serves
as the natural pacemaker (Figure 1). These nodal cells manifest spontaneous depolarizations and are
thus responsible for generating the normal cardiac rhythm; such a heart rate can also be described as
intrinsic or automatic. Importantly, the frequency of this earliest cardiac depolarization is well
modulated by both sympathetic and parasympathetic efferent innervation. In addition, the nodal rate
can also be modulated by local changes within perfusion and/or the chemical environment (i.e.,
neurohormonal, nutritional, oxygenation, etc.). Although the atrial rhythms normally emanate from the
sinoatrial node, variations in the initiation site of atrial depolarization have been documented
outside of the histological nodal tissues, particularly when high atrial rates are elicited, and may
include paranodal tissue [10-14].
One of the most conspicuous features of sinoatrial nodal cells is that they possess poorly developed
contractile apparati (a common feature to all myocytes specialized for conduction), comprising only
about 50% of the intracellular volume [1,10,15]. In general, although it typically cannot be seen
grossly, the location of the sinoatrial node is on the roof of the right atrium at the approximate
junction of the superior vena cava, the right atrial appendage, and the sulcus terminalis. In the
adult human, the node is approximately 1 mm below the epicardium, 10-20 mm long, and up to 5 mm thick
[1,16].
|
|
|
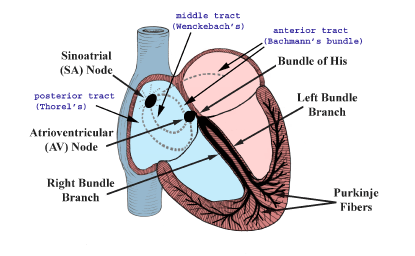
Figure 1. The conduction system of the heart. Normal excitation originates in the sinoatrial (SA) node, then propagates through both atria (internodal tracts shown as dashed lines). The atrial depolarization spreads to the atrioventricular (AV) node, passes through the bundle of His (not labeled), and then to the Purkinje fibers which make up the left and right bundle branches; subsequently all ventricular muscle becomes activated.
|
|
After initial sinoatrial nodal excitation, depolarization spreads throughout the atria. The exact
mechanisms involved in the spread of impulses (excitation) from the sinoatrial node across the atria
are still today, somewhat controversial [1,17]. However, it is generally accepted that: 1) the spread
of depolarizations from nodal cells can go directly to adjacent myocardial cells; and 2)
preferentially ordered myofibril pathways allow this excitation to rapidly transverse the right
atrium to both the left atrium and the atrioventricular node (Figure 1). It is believed by many that
there are three preferential anatomic conduction pathways from the sinoatrial node to the
atrioventricular node [1,18]. In general, these can be considered as the shortest electrical routes
between the nodes. Note that there are microscopically identifiable structures, appearing to be
preferentially oriented fibers, that provide a direct node-to-node pathway. In some hearts, pale
staining Purkinje-like fibers have also been reported in these regions. More specifically, the
anterior tract is described as extending from the anterior part of the sinoatrial node, bifurcating
into the so-called Bachmann's bundle which importantly delivers impulses to the left atrium and
with a second tract that descends along the interatrial septum that connects to the anterior part of
the atrioventricular node. The middle (or Wenckebach's pathway) extends from the superior part
of the sinoatrial node, runs posteriorly to the superior vena cava, then descends within the atrial
septum, and may join the anterior bundle as it enters the atrioventricular node. The third pathway is
described as being posterior (Thorel's) which, in general, is considered to extend from the
inferior part of the sinoatrial node, passing through the crista terminalis and the Eustachian valve
past the coronary sinus to enter the posterior portion of the atrioventricular node. In addition to
excitation along these preferential conduction pathways, general excitation spreads from cell to cell
throughout the entire atrial myocardium via the specialized connections between cells, the gap
junctions, that typically exist between all myocardial cell types (see below).
It then follows that towards the end of atrial depolarization, the excitation reaches the
atrioventricular node via the aforementioned atrial routes, with the final result being excitation of
the atrioventricular node. Further, these routes are known as the slow or fast pathways, which are
considered to be functionally and anatomically distinct. The slow pathway typically crosses the
isthmus between the coronary sinus and the tricuspid annulus; it has a longer conduction time, but a
shorter effective refractory period. The fast pathway is commonly a superior route, emanating from
the interatrial septum, and has a faster conduction rate but, in turn, a longer effective refractory
period. Normal conduction during sinus rhythm occurs along the fast pathway, but higher heart rates
and/or premature beats are often conducted through the slow pathway, since the fast pathway may be
refractory at these rates.
Though the primary function of the atrioventricular node may seem simple, that is to relay conduction
between the atria and ventricles, its structure is very complex [1]. As a means to describe these
complexities, mathematical arrays and finite element analysis models have been constructed to
elucidate the underlying structure-function relationship of the node [19].
|
In general, the atrioventricular node is located in the so-called floor of the right atrium, over the
muscular part of the interventricular septum, inferior to the membranous septum: i.e., within the
triangle of Koch, which is bordered by the coronary sinus, the tricuspid valve annulus along the
septal leaflet, and the tendon of Todaro (Figure 2). Following atrioventricular nodal excitation, the
slow pathway conducts impulses to the His bundle, indicated by a longer interval between atrial and
His activation. Currently, there is interest in the ability to place pacing leads to preferentially
activate the bundle of His; in such approaches, ultrasound or other imaging modalities are used to
map the electrical characteristic His potentials to position the pacing leads [20].
After leaving the bundle of His, the normal wave of cardiac depolarization spreads first to both the
left and right bundle branches; these pathways rapidly and simultaneously carry depolarization to the
apical regions of both the left and right ventricles (see Figure 1). Finally, the signal broadly
travels through the remainder of the Purkinje fibers and ventricular myocardial depolarization
spreads.
In certain pathological conditions, direct accessory connections from the atrioventricular node and
the penetrating portion of the bundle of His to the ventricular myocardium have been described [21].
Yet, the function and prevalence of these connections, termed Mahaim fibers, is poorly understood. A
rare bundle of Kent, an additional aberrant pathway when present, exists between the atria and
ventricles and is associated with the clinical manifestation of ventricular tachycardias (also known
as Wolff-Parkinson-White syndrome). Therapeutically, this accessory pathway is electrically
identified and then commonly ablated as a curative procedure.
The left bundle branch splits into fascicles as it travels down the left side of the ventricular
septum just below the endocardium. Its fascicles extend for a distance of 5 to 15 mm, fanning out
over the left ventricle. Importantly, typically about midway to the apex of the left ventricle, the
left bundle separates into two major divisions, the anterior and posterior branches (or fascicles).
These divisions extend to the base of each papillary muscle as well as the adjacent myocardium. In
contrast, the right bundle branch continues inferiorly, as if it were a continuation of the bundle of
His, traveling along the right side of the muscular interventricular septum. This bundle branch runs
proximally, just beneath the endocardium, and its course runs slightly inferior to the septal
papillary muscle of the tricuspid valve before dividing into fibers that spread throughout the right
ventricle. The complex network of conducting fibers that extends from either the right or left bundle
branches is composed of the rapid conduction cells known as Purkinje fibers. Purkinje fibers in both
the right and left ventricles act as preferential conduction pathways to provide rapid activation, so
to coordinate the excitation pattern within the various regions of the ventricular myocardium. Most
of these fibers travel within the trabeculations of the right and left ventricles, as well as within
the myocardium itself. Due to tremendous variability in the degree and morphology of the
trabeculations existing both within and between species, it is likely that variations in the left
ventricular conduction patterns also exist. It should be noted that one of the most common and easily
recognized conduction pathways found in mammalian hearts is the moderator band, which contains
Purkinje fibers from the right bundle branch (see:
http://www.vhlab.umn.edu/atlas/right-ventricle/moderator-band/index.shtml). Furthermore, in many
human hearts, within both the right and left ventricles, one can identify conduction bands that are
white in appearance (e.g., see apex videos within the right and left ventricles).
In 1910, Aschoff and Monckeberg provided three criteria for considering a myocardial cell as a
specialized conduction cell, including: 1) the ability to histologically identify discrete features;
2) the ability to track cells from section to section; and 3) insulation of the cell by fibrous
sheaths from the nonspecialized contractile myocardium [22,23]. It is noteworthy that only the cells
within the bundle of His, left and right bundle branches, and Purkinje fibers satisfy all three
criteria. No structure within the atria meets all three criteria, including the Bachmann's
bundle, sinoatrial node, and atrioventricular node (which are all uninsulated tissues). Yet, with
major advances in histo-molecular techniques, it is likely that new criterion will follow that better
define the uniqueness of specialized conduction structures.
|
|
|
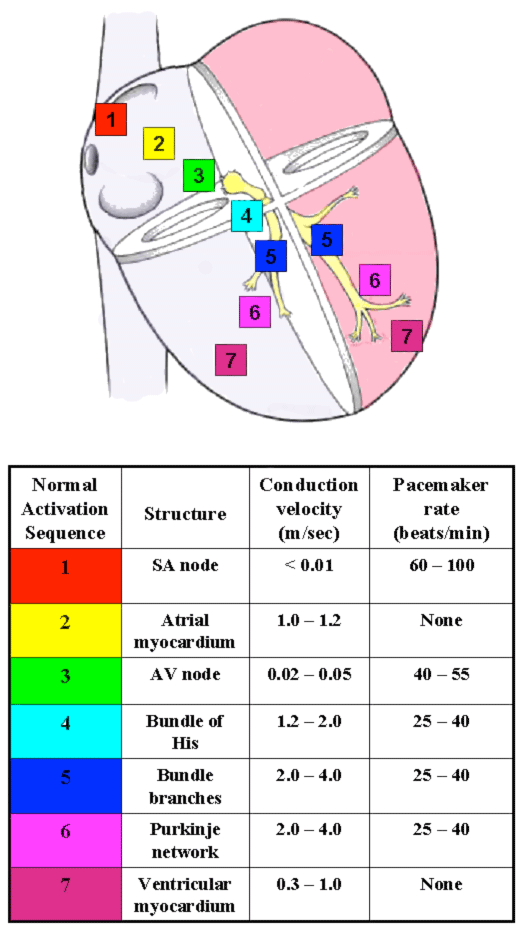
Figure 2. The conduction system of the heart. Left: Normal excitation originates in the sinoatrial (SA) node then propagates through both atria. The atrial depolarization spreads to the atrioventricular (AV) node, and passes through the bundle of His to the bundle branches/Purkinje fibers. Right: The table shows conduction velocities and intrinsic pacemaker rates of various structures within the cardiac conduction pathway. The structures are listed in the order of activation during a normal cardiac contraction, beginning with the sinoatrial node. Note that the intrinsic pacemaker rate is slower in structures further along the activation pathway. For example, the atrioventricular nodal rate is slower than the sinoatrial nodal rate. This prevents the atrioventricular node from generating a spontaneous rhythm under normal conditions, since it remains refractory at rates <55 beats per minute. If the sinoatrial node becomes inactive, the atrioventricular nodal rate will then determine the ventricular rate. Tabulation adapted from Katz AM. Physiology of the Heart, third edition. Philadelphia: Lippincott, Williams, and Wilkins, 2001.
|
|
|



